Further research are desired to investigate this method. Quite a few lines of evidence indicate that PP2, an inhibitor of non receptor tyrosine kinase c Src?a mediator of the EGFR signaling pathway?can abolish E2 induced Erk1/ 2 phosphorylation and, thus, inhibit MCF seven cell growth. In our research, GPR30 activation was inhibited by its distinct antagonist G15, hence restraining proliferation of TAM R cells by initiating apoptosis beneath Tam interven tion. These results are supported by the investigation of Ignatov et al, which indicated that GPR30 anti sense ol igonucleotides could eradicate GPR30 ligand mediated growth stimulation of TAM R cells. From the in vivo study of the proliferative prospective of GPR30, combin ation treatment of G15 plus Tam considerably decreased TAM R tumor dimension, whereas solutions with Tam or G15 alone didn’t.
GPR30 target therapy could improve apoptosis in TAM R xenografts, whereas apop tosis selleck chemical SB 525334 rates from Tam or G15 treatment don’t signifi cantly vary from that of the ethanol treated group. Synergistic interaction of GPR30 along with the EGFR sig naling pathway enhances breast cancer proliferation, which allows tumor progression in the presence of tamoxifen. Although a number of endocrine resistant breast cancer models are based on inappropriate action of the EGFR signal ing pathway, the existing model displays variable activation in the EGFR downstream cascade. Levels of phosphorylated Erk1/2 improved transiently in our TAM R cells and in long-term tamoxifen treated designs reported by other folks. In contrast, sustained Erk1/2 phosphorylation was observed in long-term estrogen deprived MCF 7 cells.
These differences may relate to approaches that breast cancer cells adapt to several endo crine solutions. selleck Despite the fact that inappropriate activation of your EGFR signaling pathway is extensively accepted being a important mechanism of tamoxifen resistance, the first aspect that transactivates EGFR is still disputed. Our study hence aimed to demonstrate the purpose of GPR30 while in the produce ment of tamoxifen resistance. In breast cancer MTs, GPR30 expression appreciably enhanced relative to cor responding PTs and correlated with EGFR expression. Endocrine treatment brought on elevated ligand dependent activation with the EGFR downstream component Erk1/2, with consequential development stimulation?which would lead breast cancer cells to create tamoxifen resistance. These phenomena were potentially linked to translocation of GPR30 for the cytomembrane and reduction of GPR30 induced cAMP manufacturing. As crosstalk between GPR30 and also the EGFR signaling pathway intensified, inhibited GPR30 activity could advertise apoptosis 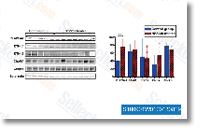 initi ation in drug resistant cells while in the presence of tamoxifen.
initi ation in drug resistant cells while in the presence of tamoxifen.
BCL-2PROTEIN
The over-expression of the anti-apoptotic Bcl-2 protein in lymphocytes
